Global Central Lab Market Based on Services-(Genetic Services, Microbiology Services, Special Chemistry Services, Biomarker Services, Other Services) End-User-(Pharmaceutical Companies, Academic & Research Institutes, Biotechnology Companies) by Region and Companies - Industry Segment Outlook, Market Assessment, Competition Scenario, Trends and Forecast 2023-2033
- Published date: June 2024
- Report ID: 83671
- Number of Pages: 368
- Format:
-
keyboard_arrow_up
Quick Navigation
Market Overview
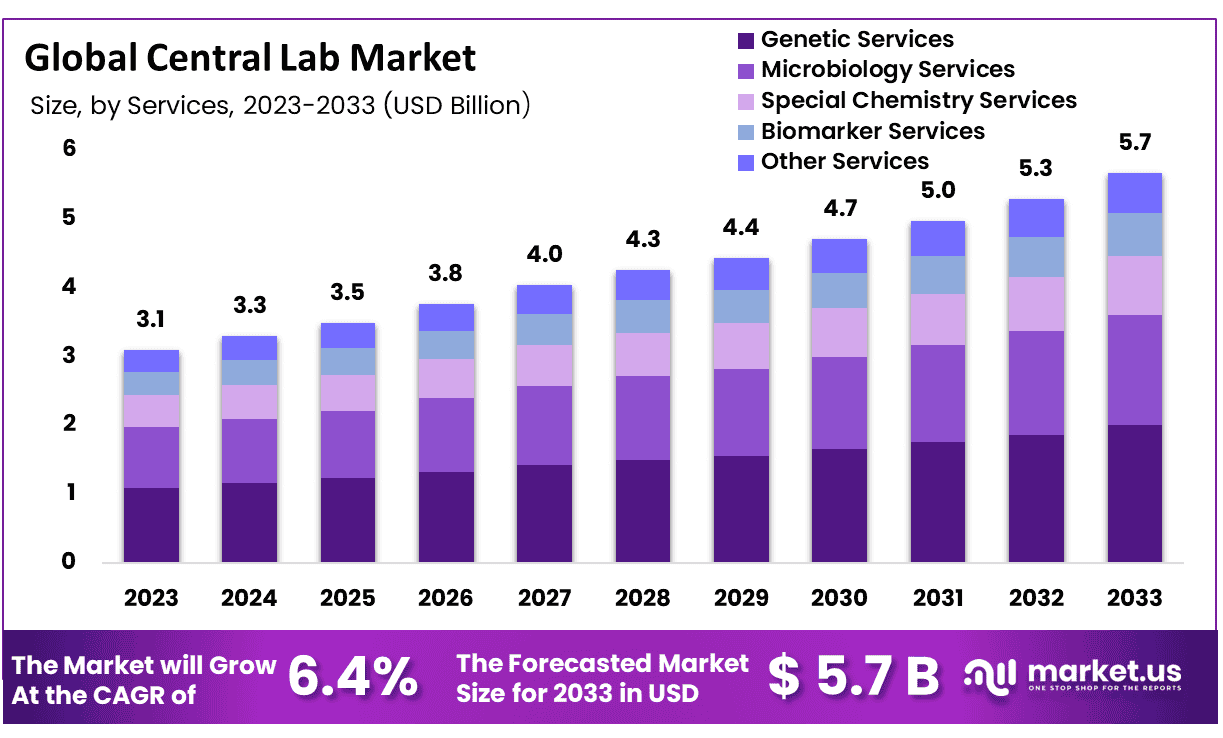
The Global Central Lab Market size is expected to be worth around USD 5.7 Billion by 2033 from USD 3.1 Billion in 2023, growing at a CAGR of 6.4% during the forecast period from 2024 to 2033
Pharmaceutical companies are always looking for strategies to cut product development and manufacturing costs. One of the most typically outsourced services by these organizations is the aspect of central lab services. The utilization of central labs not only saves money but also speeds up the medication development process. Pharmaceutical and biopharmaceutical companies typically outsource their central labs entirely. Several Contract Research Organizations (CROs) are interested in central lab services because they offer packaged services that allow them to take advantage of a wide range of services.
Bundling services and using advanced technological solutions are two main techniques that central lab market players employ. Bundled services offer a variety of services to pharmaceutical and biopharmaceutical companies at a discounted rate due to the high volume of clinical trial research data and increasing complexity. Furthermore, using advanced technology and software to automate various processes helps to reduce the time required for particular services, lowering the clinical trial’s cost in the process.
Central lab businesses have been profoundly transformed by changes in consumer demand. Consumers increasingly desire tailored approaches that meet their individual needs; personalized monitoring of candidates for certain diseases has proved particularly advantageous to customers and necessitates adjustments to logistics and sample management to accommodate this personal approach.
Key Takeaways
- Market Size & Growth: Central Lab Market size is expected to be worth around USD 5.7 Billion by 2033 from USD 3.1 Billion in 2023, growing at a CAGR of 6.4%.
- By Service Analysis: Biomarker Services accounted 37.6% revenue share in 2023 .
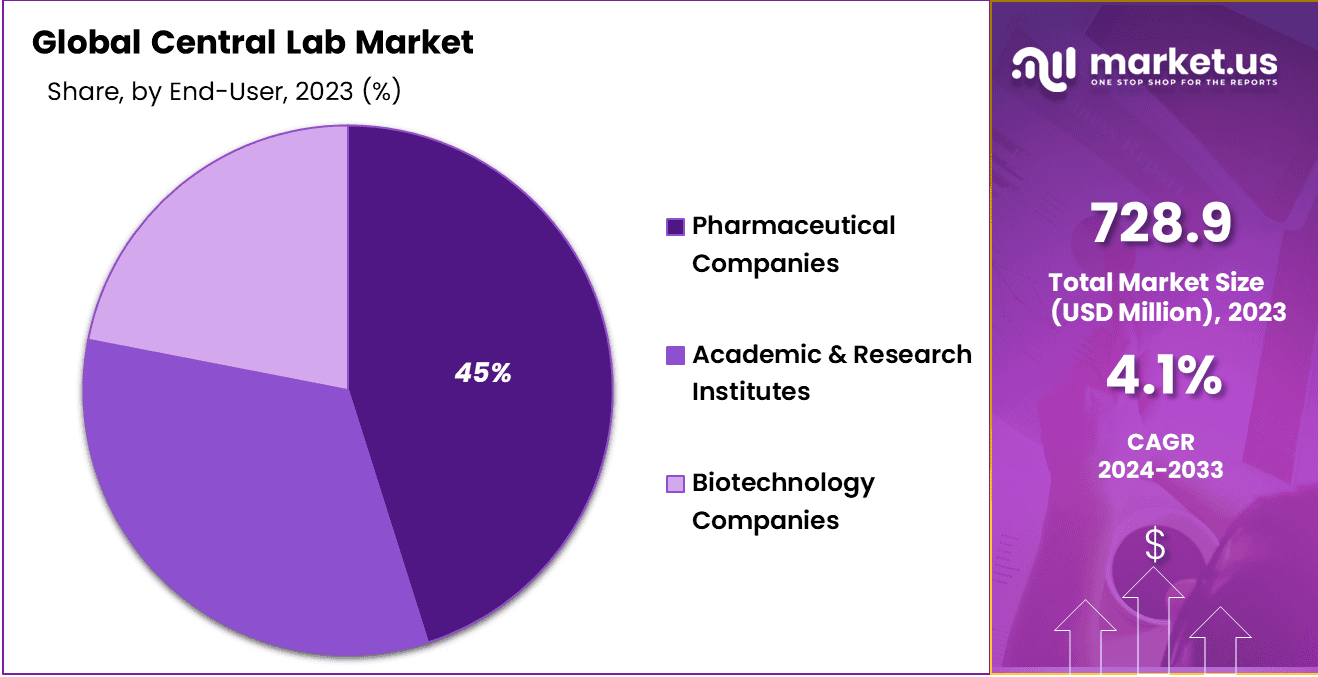
- By End-User Analysis: Pharmaceutical companies’ segment dominated the market in 2023 with 45% market share.
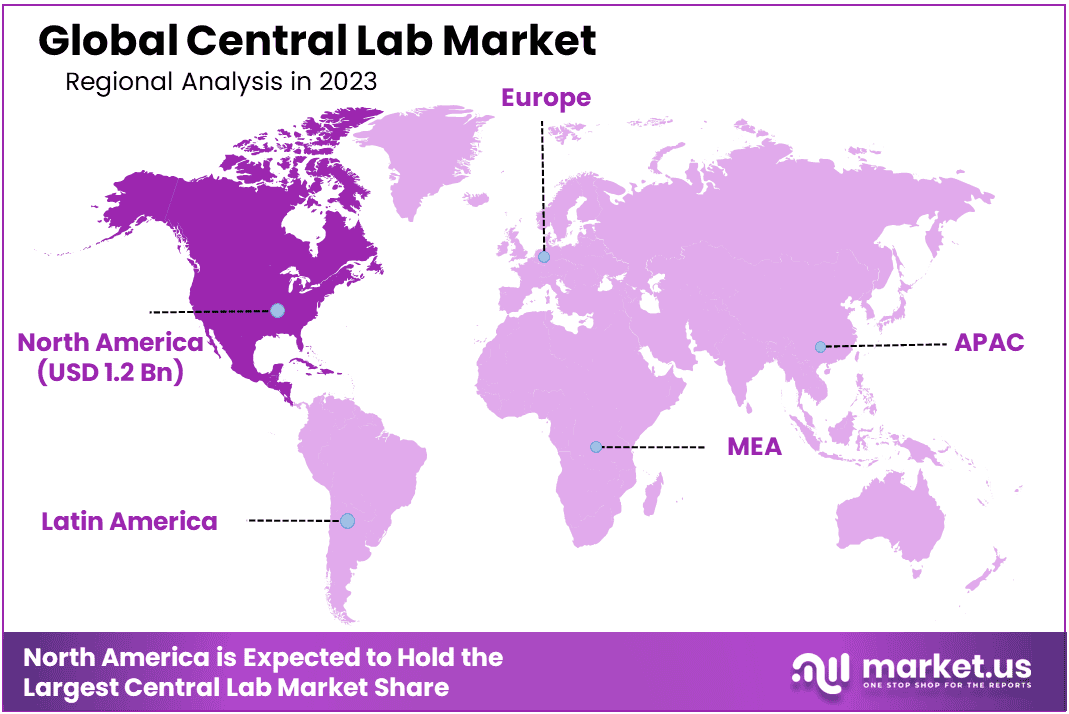
- Regional Analysis: North American region accounted for 38.7% and holding a USD 1.2 Billion value for the global central labs market.
- Technological Integration: Advances in automation, digital pathology, and AI are enhancing diagnostic accuracy and lab efficiency.
- Regulatory Compliance: Adherence to CLIA and CAP standards is crucial, impacting market operations significantly.
- Challenges and Opportunities: High technology costs and multi-site trial complexities are challenges, while personalized medicine and emerging markets offer growth opportunities.
By Service Analysis
Based on services, the market is divided into various segments, including Genetic Services, Microbiology Services, Special Chemistry Services, Biomarker Services, Other Services. Biomarker Services accounted 37.6% revenue share in 2023. Central labs are the preferred choice for conducting clinical research and trials, primarily because these endeavors are both time-consuming and expensive.
Pharmaceutical companies typically lack the necessary infrastructure and expertise to independently carry out clinical studies. Consequently, they opt to outsource these tasks to central labs, which handle a range of clinical studies aimed at developing and bringing new drugs or vaccines to market. The selection of central labs is further driven by the advanced technology they offer, ensuring timely and error-free results for various clinical trial services.
By End-User Analysis
Pharmaceutical companies’ segment dominated the market in 2023 with 45% market share. Central labs played an essential role in supporting clinical trials for new pharmaceuticals as well as genetic testing services for conditions like Alzheimer’s, sickle cell anemia and Parkinson’s.
Central labs are expected to experience continued growth over the forecast period as more clinical studies and genetic illnesses emerge and cost-cutting measures taken by pharmaceutical companies will drive increased demand for central labs – further expanding market growth potential in 202-2033.
Importance
The technological requirements needed to transfer clinical trial samples highlight the importance of a central lab. Two important strategies adopted by players in the global central lab market are the utilization of modern technical solutions and service bundling. This market is expected to profit from the presence of a large volume of clinical trial data for study and increasing complexities. Bundled services offer biopharmaceutical and pharmaceutical firms a wide range of services at a much lower cost.
The global central lab market is expanding at a rapid pace, with a growing number of hospitals coming to realize the benefits of outsourcing their lab testing needs. Even though it may cost more initially, hospitals are gradually figuring out that a central lab will save them money in the long run. Remote labs have been able to provide quick turnaround times for routine tests while offering lower prices on fewer routine tests.
The global central lab market is thriving as a result of the need for clinical laboratory testing. The demand for rapid, convenient, and inexpensive testing has emerged, particularly in developing countries where there are an increasing number of people who can afford to pay for these services.
Central Labs are known for its advanced technology and state-of-the-art equipment. A central lab offers a myriad of services, such as testing, consultation, laboratory management, specimen management, analytical testing, research, education, and an on-site pharmacy. Central labs are also able to offer different types of equipment which is more difficult for many smaller labs to have.
Market Segments
Services
- Genetic Services
- Microbiology Services
- Special Chemistry Services
- Biomarker Services
- Other Services
End-User
- Pharmaceutical Companies
- Academic & Research Institutes
- Biotechnology Companies
Driver
One of the primary factors driving growth in the central lab market is technological progress in diagnostic technologies. Healthcare has undergone an enormous transition toward precision medicine and personalized treatments approaches, creating opportunities in central lab markets worldwide.
Central laboratories play a pivotal role in this transformation by providing sophisticated diagnostic tools and techniques. Central labs now boast cutting-edge technologies such as next-generation sequencing (NGS), digital PCR and advanced imaging systems that enable more precise and comprehensive diagnostic services, not only improving patient care by facilitating early disease detection but also supporting pharmaceutical and biotechnology industries with conducting precise clinical trials.
Trend
One prominent trend driving central lab market growth is pharmaceutical and biotechnology companies’ growing trend of outsourcing clinical trial services from central labs. Outsourcing allows these firms to focus on their core competencies while drawing upon central lab expertise for testing trials.
Central labs offer services like sample processing, bioanalytical testing and data management that are essential components of clinical trials; driving this trend further is the need for cost-effective yet specialized services as the complexity of trials increases.
Restraint
Even as the central lab market experiences growth, it faces unique obstacles. One such hindrance is compliance with stringent regulatory requirements governing clinical testing and laboratory operations – such as Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) compliance is essential to guaranteeing the quality and integrity of services provided by central labs; meeting such standards often necessitates substantial investments in infrastructure, staff training programs and quality assurance measures that may act as barriers to entry for new players looking to enter this market.
Opportunity
An attractive opportunity for central lab markets lies in the growing acceptance of precision medicine approaches in patient care. Precision medicine caters medical treatments and interventions specifically to each individual based on factors like genetics, lifestyle and environment; central labs play an integral part in supporting this form of care by offering advanced diagnostic testing services like genomic sequencing and molecular profiling; with demand increasing for personalized treatments options increasing, central labs may expand their service offerings accordingly.
Regional Analysis
In 2023, the North American region accounted for 38.7% and holding a USD 1.2 Billion value for the global central labs market, thanks to rapid technological development, its well-developed healthcare infrastructure in both countries, and an influx of pharmaceutical companies based in this region. Furthermore, rapid approval processes for new drugs has contributed significantly to North American market expansion.
At first glance, Asia Pacific seems poised for rapid expansion during the forecast period. This can be attributed to rising popularity of clinical studies conducted in China as well as rising laboratory numbers across this region. Furthermore, this region stands out due to relatively lower workforce costs; these characteristics provide it with an edge and help boost market expansion.
Key Regions
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Key Players Analysis
The report highlights key players within the central lab market and includes chapters devoted to competitive landscape and company profiles for this segment. Analyses of key players in the market focus on their financial statements, major developments, strategic approaches to market entry, geographic footprint and various other crucial aspects.
This chapter also covers strengths and weaknesses, opportunities risks, and opportunities (SWOT analysis), winning essentials, current strategy & focus as well as threats from competitors for companies who rank among the best three or five on the market. Furthermore, companies included can be modified according to client requirements.
Кеу Рlауеrѕ
- A.P. Møller Holding A/S
- Labconnect
- Lambda Therapeutics Research Ltd
- Cirion Biopharma Research Inc.
- Cerba Research
- Ampersand Capital Partners
- Medicover Integrated Clinical Services
- ACM Global Laboratories
- A.P. Møller Holding A/S
- Eurofins Scientific
- Other Key Players
Recent Developments
- A.P. Møller Holding A/S (March 2024): A.P. Møller Holding A/S acquired a majority stake in Nordic BioTech Labs, enhancing its portfolio in biopharma research services and expanding its presence in the European market. This strategic move aims to leverage Nordic BioTech’s expertise in cutting-edge laboratory solutions.
- Labconnect (April 2024): Labconnect launched its new Digital Pathology Platform, integrating AI-driven diagnostic tools to enhance precision and speed in clinical trials. This innovation is set to streamline workflows and improve data accuracy, benefiting researchers and healthcare providers globally.
- Lambda Therapeutics Research Ltd (February 2024): Lambda Therapeutics Research Ltd merged with MedCore Labs, consolidating their resources to strengthen their global clinical trial services. The merger aims to provide comprehensive laboratory and research solutions, enhancing efficiency and expanding their market reach.
- Cirion Biopharma Research Inc. (January 2024): Cirion Biopharma Research Inc. acquired BioAssure Labs, a move to broaden its service offerings in bioanalytical and immunogenicity testing. This acquisition enhances Cirion’s capacity to support complex clinical trials and regulatory requirements.
- Cerba Research (May 2024): Cerba Research introduced its Global Lab Network Platform, designed to facilitate seamless coordination and data sharing across international clinical trial sites. This platform aims to improve the efficiency and reliability of multi-center trials.
Report Scope
Report Features Description Market Value (2023) USD 3.1 Billion Forecast Revenue (2033) USD 5.7 Billion CAGR (2024-2033) 6.4 % Base Year for Estimation 2023 Historic Period 2018-2022 Forecast Period 2024-2033 Report Coverage Revenue Forecast, Market Dynamics, Competitive Landscape, Recent Developments Segments Covered Services-(Genetic Services, Microbiology Services, Special Chemistry Services, Biomarker Services, Other Services) End-User-(Pharmaceutical Companies, Academic & Research Institutes, Biotechnology Companies) Regional Analysis North America-US, Canada, Mexico;Europe-Germany, UK, France, Italy, Russia, Spain, Rest of Europe;APAC-China, Japan, South Korea, India, Rest of Asia-Pacific;South America-Brazil, Argentina, Rest of South America;MEA-GCC, South Africa, Israel, Rest of MEA Competitive Landscape A.P. Møller Holding A/S, Labconnect, Lambda Therapeutics Research Ltd, Cirion Biopharma Research Inc., Cerba Research, Ampersand Capital Partners, Medicover Integrated Clinical Services, ACM Global Laboratories, A.P. Møller Holding A/S, Eurofins Scientific, Other Key Players Customization Scope Customization for segments, region/country-level will be provided. Moreover, additional customization can be done based on the requirements. Purchase Options We have three licenses to opt for: Single User License, Multi-User License (Up to 5 Users), Corporate Use License (Unlimited User and Printable PDF) Frequently Asked Questions (FAQ)
What is a Central Lab in the context of the healthcare industry?A Central Lab is a specialized facility that provides comprehensive clinical testing and diagnostic services, often supporting pharmaceutical and biotechnology companies in their drug development and clinical trial processes.
How big is the Central Lab Market?The global Central Lab Market size was estimated at USD 3.1 Billion in 2023 and is expected to reach USD 5.7 Billion in 2033.
What is the Central Lab Market growth?The global Central Lab Market is expected to grow at a compound annual growth rate of 6.4%. From 2023 To 2033
Who are the key companies/players in the Central Lab Market?Some of the key players in the Central Lab Markets are A.P. Møller Holding A/S, Labconnect, Lambda Therapeutics Research Ltd, Cirion Biopharma Research Inc., Cerba Research, Ampersand Capital Partners, Medicover Integrated Clinical Services, ACM Global Laboratories, A.P. Møller Holding A/S, Eurofins Scientific, Other Key Players
Why are Central Labs crucial in the drug development process?Central Labs play a pivotal role in drug development by conducting various diagnostic tests, analyzing samples, and providing accurate data essential for evaluating the safety and efficacy of new drugs in clinical trials.
What services do Central Labs typically offer?Central Labs offer a range of services, including specimen processing, bioanalytical testing, data management, and specialized diagnostic testing, contributing to the overall success of clinical trials.
How do Central Labs contribute to precision medicine?Central Labs contribute to precision medicine by offering advanced diagnostic services, including genomic testing and molecular profiling, tailored to individual patient characteristics for more personalized and effective treatment approaches.
What are some key trends in the Central Lab Market?Notable trends include the integration of advanced technologies, global expansion, a focus on precision medicine, strategic partnerships, and increased outsourcing of clinical trials to central labs.

-
-
- A.P. Møller Holding A/S
- Labconnect
- Lambda Therapeutics Research Ltd
- Cirion Biopharma Research Inc.
- Cerba Research
- Ampersand Capital Partners
- Medicover Integrated Clinical Services
- ACM Global Laboratories
- A.P. Møller Holding A/S
- Eurofins Scientific
- Other Key Players









